What makes the specifity of a Tyrosinkinase?

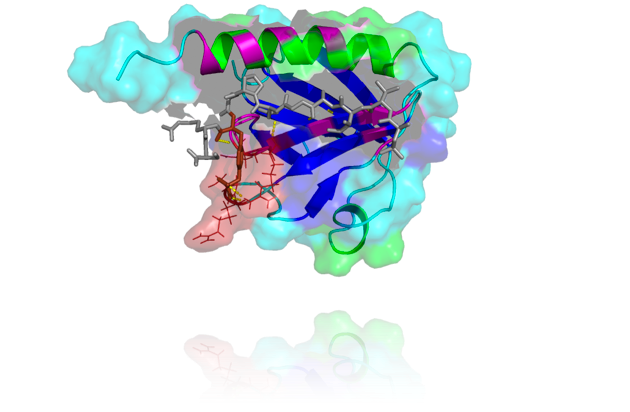
The insulin receptor (IR) and the closely related insulin-like growth factor receptor (IGF1R) are members of the transmembrane receptor tyrosine kinase family. IR autophosphorylation after insulin stimulation reveals dual activity, that is, phosphorylation of both tyrosine and serine residues. Insulin receptor kinase, if expressed as a soluble, dimeric GST-fusion protein, also displays dual activity during autophosphorylation. Dual substrate phosphorylation by IR or IGF-1R was detected only with a polylysine linker present in equimolar concentrations.
In this thesis, the effects of kinase-substrate-complexes on the dual specificity of kinases and on downstream effectors were analyzed. The phosphotyrosine binding domain (PTB) of the insulin receptor substrate-1 (IRS-1) was used as a model substrate. PTB functions as both a linker and a suitable substrate for the kinase domain of the IR and the IGF-1R. Therefore, different variants of the PTB where cloned, purified and analyzed in kinase assays. The soluble, dimeric GST-fused kinase domain of the IGF-1R was used as a model enzyme, as well as a mutant of this kinase in which PTB binding is impaired by substitution of the essential tyrosine 950.
A stable complex formation between kinase and substrate could be demonstrated. This complex has extensive impact on downstream effector kinases. An effector kinase beyond the signalosome shows only marginal catalytic activity towards the substrate at the signalosome.
Kinase and substrate form a 1:1 complex, so that a “quasi”-intramolecular reaction appeared. Therefore, the mechanism of substrate phosphorylation within this complex does not comply with Michaelis Menten kinetics.
Complex formation between kinase and substrate not only increases kinase activity but also extends kinase specificity from tyrosine phosphorylation to serine- and threonine phosphorylation. Perturbation of complex formation on the part of either the kinase or the substrate resulted in a total loss of substrate phosphorylation.
Based on these results, a model for kinase specificity is postulated which distinguishes between protein binding by docking sites and binding of substrate amino acid residues to the catalytic cleft. This model explains the specificity of tyrosine kinases: the kinase exhibits a distinct preference for every phosphoryl acceptor within the substrate, regardless of this residue being a tyrosine-, threonine-, or serine residue. This preference is determined by the characteristics of the kinase-substrate-complex, particularly the duration of the binding of the docking substrate in complex, as well as the flexibility of the phosphoryl acceptor residues.


