 |
|
|
 |
|
|
 |
 |
|
Dr. Alexander M. Reugels
Cell polarity and spindle orientation during early neurogenesis in Danio rerio.
Proper spatial and temporal specification of cells during development is crucial for the generation of cellular diversity in the nervous system of complex organisms. One way to achieve cell diversification is by means of asymmetric cell divisions where the two daughter cells adopt different fates. Such divisions can involve extrinsic and/or intrinsic factors. Extrinsic factors such as the ligand Delta and its receptor Notch act via cell-cell signalling to specify distinct cell fates: Delta activates Notch in adjacent cells, which finally directs them into alternative developmental pathways. Intrinsic factors are cell fate determinants, such as the Drosophila transcription factor Prospero or the membrane-associated protein Numb (Nb), which are localized asymmetrically in dividing cells and hence are segregated to only one daughter cell, enabling this cell to adopt a different fate from that of its sibling.
We are interested in the mechanisms underlying the establishment of cellular polarity and the generation of neuronal cell lineages during neurulation in Danio rerio (Fig. 1). We were able to show that neurulation in zebrafish embryos is characterised by oriented cell divisions and the progressive establishment of cellular polarity. Mitoses in the neural plate and neural tube are planar, but in the neural keel/rod stage the mitotic spindle rotates by 90°, causing cell divisions to occur perpendicular to the plane of the neuroepithelium. However, the mechanisms and molecules that establish cellular polarity and cause the stereotypic orientation of the mitotic spindle during neurulation are still largely unknown.
In order to address this topic, we are currently analyzing the putative cell fate determinant Numb (Fig. 2) and the role of the Par-3/Par-6/aPKC (Fig. 3) complex in setting up apicobasal polarity and specifying the orientation of the mitotic spindle. A major aspect of our work is the in vivo time-lapse imaging of GFP-tagged fusion proteins via confocal laser scanning microscopy.
|
|
|
 |
 |
|
Figure 1:
The zebrafish, like other teleosts, undergoes secondary neurulation (the following is a brief description of neurulation at the level of the 1st to 5th somite; Geldmacher-Voss et al., 2003):
In the course of convergence movements that follow gastrulation, the neural plate folds inward at the midline, between the 6- and 10-somite stages (13.3 hpf), to form the neural keel. During the 10- to 14-somite stage, the keel progressively rounds up, forming the neural rod -- a massive cellular conglomerate without a lumen -- which is finally overlaid by the adjacent epidermis at about 16 hpf. The neurocoel, the lumen of the neural tube, forms secondarily by cavitation of the neural rod, as the neuroepithelial cells retract their apical processes from the midline. Neurocoel formation starts in the 17- to 18-somite embryo, beginning ventrally in the spinal cord and progressing towards dorsal levels, and is completed by the 30-somite stage (approximately 24 hpf). Throughout neurulation the zebrafish neuroepithelium remains essentially pseudostratified, consisting of columnar cells that extend apically from the basement membrane. As in many other epithelia, neuroepithelial cells round up and divide apically (see also Papan and Campos-Ortega, Dev. Genes Evol. (Roux's Arch. Dev. Biol.) 1994 Jan;203(4):178-186).
|
|
 |
|
|
 |
 |
 |
 |
 |
|
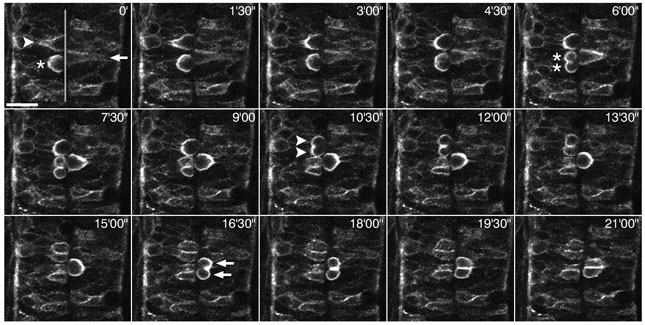
Figure 2:
We cloned two isoforms of zebrafish Numb that differ by the presence of 11 additional amino acids within the PTB domain, and identified the Numb-related protein Numblike. In order to analyze their localization in dividing neuroepithelial cells during neurulation in vivo, we injected mRNA encoding either of the Numb isoforms, or Numblike, fused to EGFP. These analyzes revealed that Numb isoform 1, which has the 11-amino acid insertion in the PTB domain (PTB-L form), is localized to the cell membrane in dividing cells, whereas both the Numb isoform 3 without the insertion (PTB-S form) and Numblike (which also has a PTB-S-type PTB domain) are localized in the cytoplasm. Time-lapse analyzes showed that the PTB-L form is distributed ubiquitously around the cell cortex of dividing cells during the neural plate and keel stages, but becomes localized to the basolateral membrane of some dividing cells during the transition from the rod to the tube stage (Reugels et al., 2006):
A sequence of fifteen confocal micrographs of a dorsal view of the neural tube of a ~24 hpf old zebrafish embryo injected with numb(PTBL PRRL):egfp mRNA. The transparent grey line in the first picture marks the neurocoel. Scale bar: 20 um. Three cells (arrows, arrowheads and asterisks in 0’) one after another round up at the neurocoel and subsequently undergo mitotic cell division. Note that Numb:EGFP becomes localized to the basolateral cell cortex in all three cells, whereas the apical side is completely devoid of signal. Although Numb:EGFP is clearly asymmetrically localized in these cells, it is distributed to both daughter cells upon division, since the cells divide parallel to the plane of the neuroepithelium (for further information on the localization of Numb:EGFP see Reugels et al., 2006).
|
|
|
 |
|
|
|
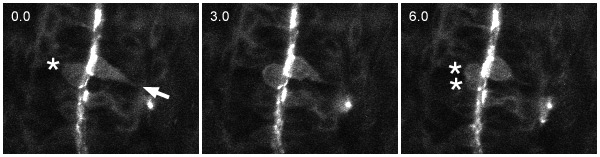
Figure 3:
Cell polarity is established progressively during neurulation in zebrafish embryos, as revealed by investigations of the localization of several characteristic apical markers such as ASIP/PAR-3:EGFP, aPKC, beta-Catenin and ZO-1. ASIP/PAR-3:EGFP, for example, is distributed diffusely at the plasma membrane of neuroepithelial cells in the neural plate, with little or no evidence for preferential localization at apical levels. Localization at the apical pole of the cells first appears during the neural keel/rod stage, and becomes quite obvious in the neural tube (Geldmacher-Voss et al., 2003):
A sequence of three confocal micrographs of a dorsal view of the neural tube of a ~24 hpf old zebrafish embryo injected with asip/par-3:egfp mRNA. Two cells are visible, one of which is dividing (asterisks), whereas the other one is rounding up at the midline prior to cell division (arrow). Note that ASIP/PAR-3:EGFP is localized at the apical membrane of dividing and non-dividing neuroepithelial cells in the neural tube (for further information on the localization of ASIP/PAR-3:EGFP see von Trotha et al., 2006).
|
 |
 |
|
Selected Publications:
 Alexandre, P., Reugels, A.M., Barker, D., Blanc, E., Clarke, J.D. Daughter cell fate is non-stochastic and neurons are generated from the apical daughter in asymmetrically fated divisions in the zebrafish neural tube. Submitted to Nat. Neurosci. July 2009. Alexandre, P., Reugels, A.M., Barker, D., Blanc, E., Clarke, J.D. Daughter cell fate is non-stochastic and neurons are generated from the apical daughter in asymmetrically fated divisions in the zebrafish neural tube. Submitted to Nat. Neurosci. July 2009.
 Tawk, M., Araya, C., Lyons, D.A., Reugels, A.M., Girdler, G.C., Bayley, P.R., Hyde, D.R., Tada, M., Clarke J.D. A mirror-symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature. 2007 Apr 12; 446(7137):797-800. Tawk, M., Araya, C., Lyons, D.A., Reugels, A.M., Girdler, G.C., Bayley, P.R., Hyde, D.R., Tada, M., Clarke J.D. A mirror-symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature. 2007 Apr 12; 446(7137):797-800.
 von Trotha, J. W., Campos-Ortega, J. A. and Reugels, A. M. Apical localization of ASIP/PAR-3:EGFP in zebrafish neuroepithelial cells involves the oligomerization domain CR1, the PDZ domains and the C-terminal portion of the protein. Dev. Dyn. 2006 Apr;235(4):967-977. von Trotha, J. W., Campos-Ortega, J. A. and Reugels, A. M. Apical localization of ASIP/PAR-3:EGFP in zebrafish neuroepithelial cells involves the oligomerization domain CR1, the PDZ domains and the C-terminal portion of the protein. Dev. Dyn. 2006 Apr;235(4):967-977.
 Reugels, A. M., Boggetti, B., Scheer, N. and Campos-Ortega, J. A. Asymmetric localization of Numb:EGFP in dividing neuroepithelial cells during neurulation in Danio rerio. Dev. Dyn. 2006 Apr;235(4):934-948. Reugels, A. M., Boggetti, B., Scheer, N. and Campos-Ortega, J. A. Asymmetric localization of Numb:EGFP in dividing neuroepithelial cells during neurulation in Danio rerio. Dev. Dyn. 2006 Apr;235(4):934-948.
 Geldmacher-Voss, B., Reugels, A. M., Pauls, S. and Campos-Ortega, J. A. A 90 degree rotation of the mitotic spindle changes the orientation of mitoses of zebrafish neuroepithelial cells. Development. 2003 Aug;130(16):3767-80. Geldmacher-Voss, B., Reugels, A. M., Pauls, S. and Campos-Ortega, J. A. A 90 degree rotation of the mitotic spindle changes the orientation of mitoses of zebrafish neuroepithelial cells. Development. 2003 Aug;130(16):3767-80.
 Complete list of publications (search Pubmed for: Reugels-AM). Complete list of publications (search Pubmed for: Reugels-AM).
Contact:
|
 |
|
|
 |
|
 |
|
|
 |
|