Research Aims
Elucidate how ubiquitylation of mitofusins regulates mitochondrial fusion and impacts cellular performance.
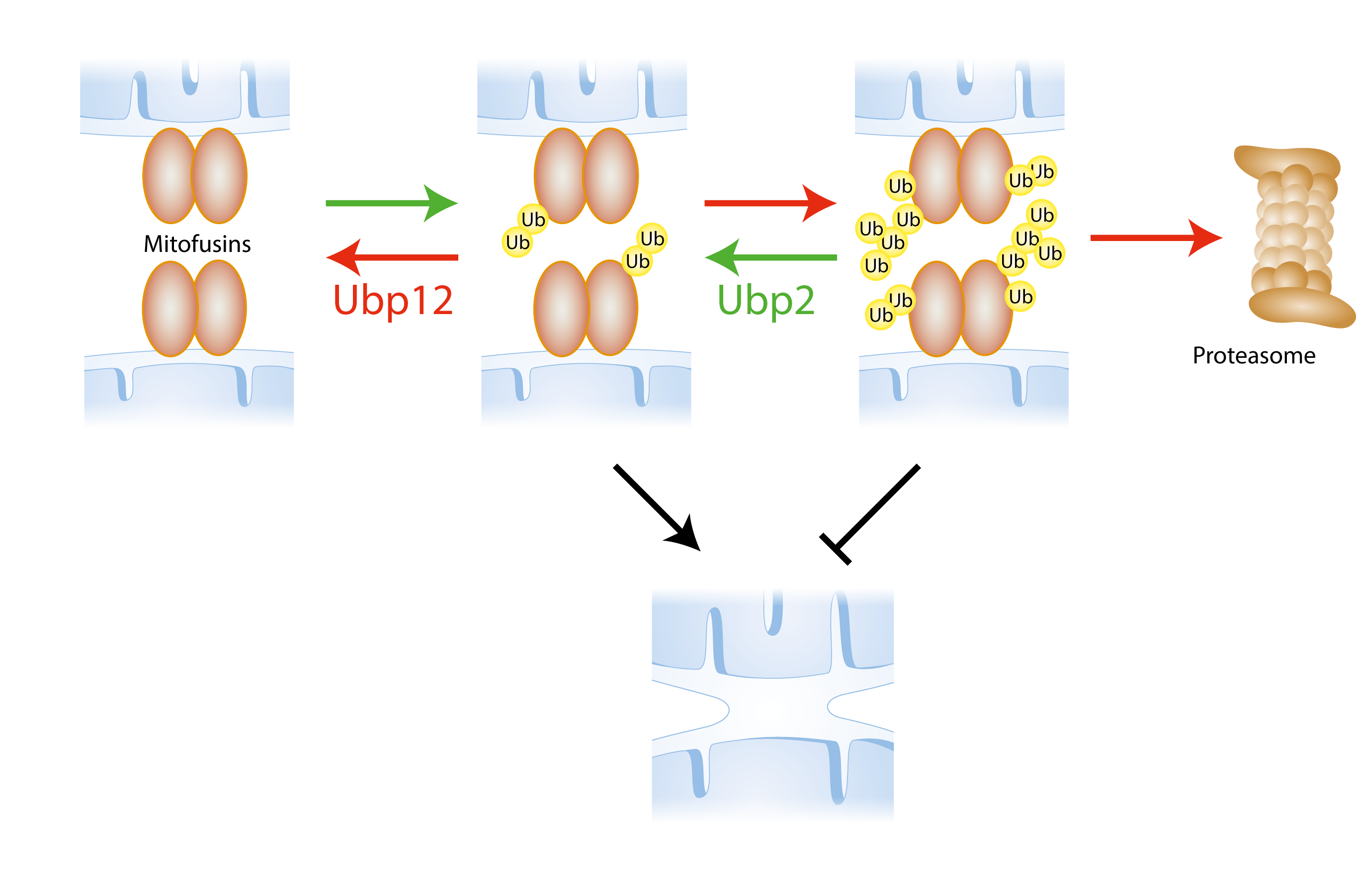
Fusion of the mitochondrial outer membrane (OM) is mediated by the ubiquitylation of mitofusins, resident in the OM and facing the cytoplasm. The ubiquitlyated status of mitofusins needs to be highly regulated as massive ubiquitylation leads to their degradation by the proteasome. We identified two DUBs responsible for the deubiquitylation of mitofusins, called Ubp12 and Ubp2. These DUBs fine tune the ubiquitylated status of mitofusins in opposing ways: Ubp12 removes ubiquitin forms from mitofusins that promote mitochondrial fusion (making Ubp12 an anti-fusion DUB), whereas Ubp2 removes ubiquitylation from mitofusins that inhibit fusion (pro-fusion DUB). Moreover, Ubp12 an Ubp2 also affect cellular ubiquitin homeostasis in an opposing manner.
Regulation of mitochondrial fusion by ubiquitylation of mitofusins. Mitofusins are ubiquitylated, which allows for mitochondrial outer membrane fusion. Ubiquitin is removed by DUBs (Ubp12 and Ubp2) with opposing effects on the fusion process. Green arrows = processes that promote fusion; Red arrows = processes that inhibit fusion.
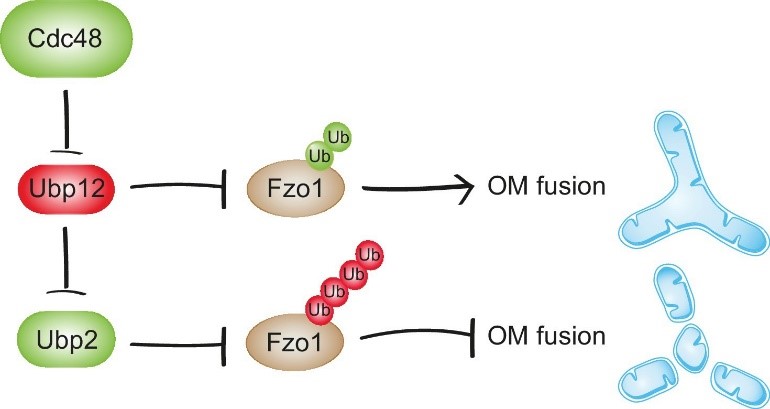
We now investigated how these two deubiquitylases, which perform an identical task (removing ubiquitin), on the same target (Fzo1), can exert opposing effects on mitochondrial fusion. This is important to know, because mitochondrial dynamics allows metabolic adaptation to the cellular environment. We found that Ubp12 represses Ubp2, meaning that activation and inhibition of fusion are coordinated. This explains the opposite roles of these two deubiquitylases on Fzo1 function. Moreover, it clearly demonstrates a hierarchical organization among deubiquitylases. Mechanistically, this is possible because Ubp12 and Ubp2 are themselves also ubiquitylated. In addition to Ubp2 and Ubp12, we also identified Cdc48 as the master regulator of this deubiquitylase cascade. Cdc48 is an essential ubiquitin-dependent ATPase chaperone. We found that Cdc48 promotes degradation of Ubp12, limiting its activity. This led to the identification of a novel regulatory pathway that allows fine-tuning of the fusogenic activity of Fzo1.
Cdc48 activates mitochondrial fusion via Ubp12 and Ubp2. In contrast, Cdc48 impairment blocks the repression of Ubp12. Ubp12 then leads to a cascade of events inhibiting mitochondrial fusion: A) removal of the pro-fusion ubiquitylated forms and B) inhibition of Ubp2, consequently leading to the accumulation of the anti-fusion ubiquitylated forms. This cascade allows a synergistic effect of Cdc48, via a DUB regulatory cascade, to effectively promote or inhibit mitochondrial fusion.