Department of Cell Genetics
Institute for Genetics - University of Cologne
Department Head: Prof. Dr. Jonathan C. Howard
Welcome - Research Topic - Recent Publications - People - Former Members - Contact - IRG Database - Lab Database
Jonathan C. Howard: Curriculum Vitae - Publications - Research Grants
Function of the Interferon-Inducible Immunity-Related GTPases in the Innate Immune Response to Toxoplasma Gondii Infection
It is becoming increasingly clear that cell-autonomous resistance mechanisms exist that tend to counteract the replication and survival of intracellular pathogens. These resistance programs are not restricted to cells of the classical immune system like B-cells, T-cells or macrophages but are potentially active in every body cell. Such mechanisms are normally latent in uninfected or unstimulated cells but are activated by cytokines and especially interferons. Interferons are produced upon infection and are subdivided into type I and type II interferons. Type I interferons are secreted by virus infected cells while secretion of type II interferon, also called interferon-γ (IFN-γ), is restricted to T-cells, NK-cells and macrophages. The two classes of interferons induce an extraordinarily complex cellular response regulating the expression of several hundred genes.
An intracellular way of life can protect pathogens from antibodies, but hosts can deploy other specialized defense mechanisms against such pathogens. Toxoplasma gondii, is an intracellular protozoal pathogen of mammals and birds, and commonly infects humans. Mice exploit a specialized intracellular resistance system, the IFN-γ inducible immunity-related GTPases (IRG proteins, also known as p47 GTPases), for defense against T. gondii. IRG proteins accumulate rapidly on the membrane surrounding intracellular parasites. Shortly after, this membrane ruptures and the parasite dies. This process is followed by the necrotic death of the host cell. IRG proteins are GTPases, enzymes that release chemical energy by breaking down guanosine trisphosphate (GTP) in the cell. Probably, they can convert released chemical energy into the mechanical force that damages the vacuole membrane.
 |
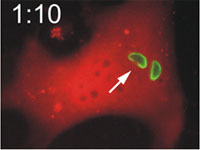 |
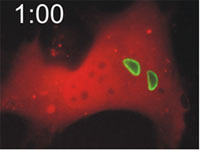
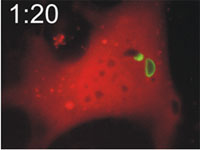
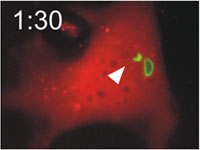
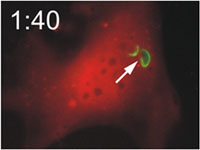
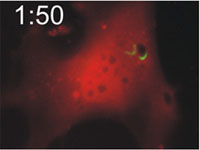
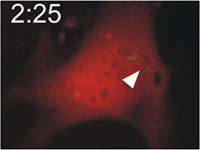
Host cells die after disruption of the parasitophorous vacuole (PV) and death of the T. gondii. Mouse embryonic fibroblasts were treated with IFN-γ and co-transfected with EGFP-tagged Irga6 (green) and soluble DsRed constructs. Cells were then infected with ME49 strain T. gondii and observed microscopically by time-lapse photography. Three PVs were observed within this cell when the experiment started. Two Irga6-EGFP-positive PVs were disrupted at 1:10 (1min 10 secs) and 1:40, respectively (arrows). These two T. gondii became permeable to DsRed (died) at 1:30 and 2:25, respectively (arrowheads). At 2:30 after infection, the DsRed signal suddenly disappeared from the host cell, indicating loss of membrane integrity and death of the cell. Zhao et al., 2009 |
 |
 |
|
 |
 |
|
 |
 |