Mechanisms regulating age-associated decline of skin regenerative capacity
A decline in skin regenerative capacity, increased skin fragility with disturbed barrier function and impaired wound healing are leading causes of increasing morbidity and mortality in the elderly. Understanding the underlying molecular and cellular mechanisms is important for the development of strategies to intervene in aging- and disease-associated loss of skin function.
The aim of this project is to understand the role of the nutrition-sensing pathway, and specifically of TOR signalling, in skin homeostasis, regeneration and aging.Rapamycin inhibits cell growth and is used in the clinic for treating diseases such as cancer. The molecular target of rapamycin, the Ser/Thr kinase TOR, senses and integrates a variety of environmental cues from nutrients and growth factors, acting as a nexus point for cellular signals to control growth and metabolism.
TOR interacts with several proteins to form two distinct complexes named TOR complex 1 (TORC1) and 2 (TORC2). Altering the activity of components of the TOR pathway either genetically or by pharmaceutical intervention can extend healthy lifespan in laboratory animals. It is not clear to what extent this is due to organ-specific or systemic effects, and indeed in some organs reduction of TOR signalling can be detrimental, and the specific roles of TOR at the cellular level are just beginning to emerge.
TOR signalling is involved in wound healing in Drosophila larvae
To test the roles of TOR in epidermal regeneration in flies we established a method for long-term immobilisation of Drosophila larvae and laser-mediated epithelial injury. We imaged wound healing in vivo over extended periods with high spatial and temporal resolution, and used the wide range of genetic tools available in Drosophila as well as pharmacological approaches to perturb TOR signalling.
To assay the role of TOR in wound healing, we first fed rapamycin to larvae. The animals carry markers that allow us to follow the wound healing process, such as fluorescently tagged markers for cell membranes, actin and other cellular components. To avoid general developmental effects of rapamycin treatment, larvae were initially raised on normal food and then given food supplemented with rapamycin for 22h prior to wounding.The treatment with rapamycin (1 to 20 µM) did not interfere with normal growth or development of the larvae. However, wound closure was significantly delayed. Whereas wounds had closed by 2 h in control animals, most wounds in animals treated with rapamycin were still open after 3 h. Thus, like in mammalian skin, wound healing in theDrosophila larval epidermis is also sensitive to rapamycin, pointing to a possible involvement of TOR signalling in epidermal regeneration in Drosophila.
TORC1 signalling regulates epidermal integrity and morphology in Drosophila
Rapamycin inhibits TOR signalling through binding and specific allosteric inhibition of TORC1. To test which of the TOR complexes was necessary for proper wound healing, we used RNA interference constructs against Raptor and Rictor, the specific core components of TORC1 and TORC2, respectively, which we expressed in the larval epidermis.
Knockdown of TORC2 had no effects on cell shape or morphology and wound healing occurred normally, showing that only the rapamycin-sensitive TORC1 is required during wound healing. Reduction of TORC1 led to serious epidermal defects and to larval or pupal lethality, consistent with an essential role of TORC1 in epidermal morphology, integrity and wound healing in Drosophilalarvae.Tissue specific activation of S6Kinase suppresses rapamycin-mediated delays of wound healing
TORC1 has two major downstream effectors, both involved in protein synthesis, S6 kinase (S6K) and its antagonist 4E-BP. To test which of these effectors are needed for wound healing we experimentally raised and lowered their levels, either alone, or in rapamycin-treated larvae.
Neither loss nor constitutive activity of 4E-BP affected wound healing , whereas reduction of S6K in the epidermis resulted in delayed wound healing. If rapamycin acts through S6K, then activation of S6K should at least partially rescue the rapamycin effect.
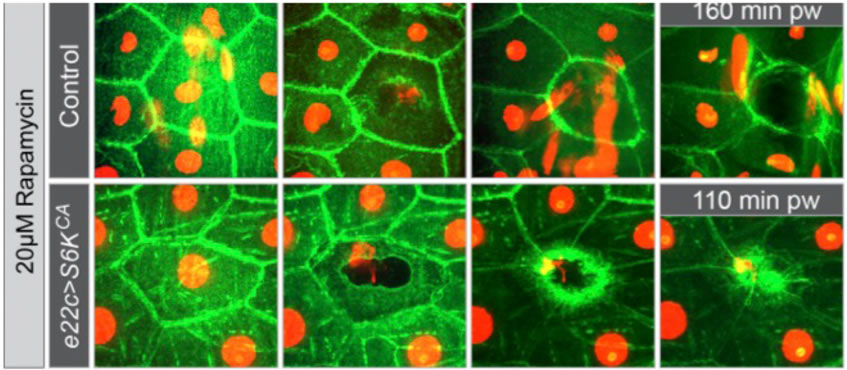
To test this we overexpressed a constitutively active version of S6K (S6KSTDETE) in the epidermis of rapamycin treated larvae. This completely suppressed the rapamycin-induced delay in wound healing (Figure 1). Thus, S6K activation downstream of TORC1 is sufficient to enable efficient wound healing, suggesting that TOR acts through S6K.
Fig. 1. Time-lapse images of wound healing in rapamycin-treated larvae with or without constitutively active S6K. Green: Src-GFP; red: DsRed2-Nuc.