

Mechanisms of Morphogenesis
Morphogenesis, the shaping of structures in a developing organism, is an aspect of differentiation that, like other differentiation events, is controlled by transcription factors and involves a variety of subcellular effector mechanisms. The first major morphogenetic event in the Drosophila embryo, an epithelial folding that leads to the invagination of the mesoderm, depends on two transcription factors, Snail and Twist. Many of their target genes are known, but those responsible for mediating the cellular changes during the invagination of the mesoderm have not been identified. One aim of our work is to establish the hierarchy of regulatory events downstream of Twist and Snail to the effector molecules that mediate morphogenesis.
A different type of morphogenetic process, the branching of cells, occurs during the development of the tracheal system which delivers oxygen to all cells of the body. The branched network of the tracheal system in the embryo initially develops according to a stereotypic programme. Later, during larval life, tracheal cells respond to the need for oxygen in the surrounding tissue by sending out long protrusions towards oxygen-starved cells. Both the embryonic and the larval events are controlled by the Drosophila FGF-homolog, Branchless. We are investigating how the subcellular events downstream of FGF signalling contribute to branch formation and what other mechanisms are involved in the localized outgrowth of branches.Genetic hierarchy of early mesoderm development:
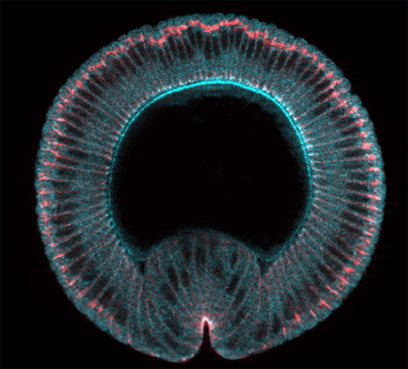
Figure: crossection through a gastrulating Drosophila embryo showing the localisation Arm/ß-catenin (red) and RhoGEFII (blue).
Many transcription factors are known that are responsible for the differentation and morphogenesis of organs or regions of developing organisms. However, in no case have the genetic cascades that control morphogenesis downstream of the transcription factors been fully elucidated. We have been studying the formation of the ventral furrow and the subsequent invagination of the mesoderm in the Drosophila embryo as an example of morphogenesis in which the first steps of the genetic regulatory cascde are extremely well understood. The mesoderm arises from a region covering most of the ventral side of the embryo which is set up under the control of the dorso-ventral patterning system of the embryo. The accumulation of high levels of the transcription factor Dorsal in nuclei on the ventral side of the embryo leads to the transcription of two genes, snail and twist, which together define the mesoderm primordium and are essential for all aspects of its differentiation and morphogenesis. Snail, a zinc-finger protein, acts mainly as a repressor of ectodermal genes in the region of the prospective mesoderm. Twist, a bHLH-protein, acts as an activator for mesodermal genes. Loss of either factor leads to specific defects in mesoderm development, loss of both to the absence of any mesodermal differentiation or morphogenesis.
No single gene has been found whose loss leads to phenotypes resembling those of either twist or snail. However, loss of function of one known target gene of Snail and Twist, folded gastrulation, causes moderate defects in mesoderm invagination. We have identified four regions in the genome whose loss results in similarly subtle and transient mutant phentypes.
For two of these, the genes responsible for the loss of function phenotypes have been identified. Both are expressed in the mesoderm primordium and are involved in controlling the cell division cycle in the mesoderm. In their absence, premature mitoses interfere with the cell shape changes that drive the invagination. We are in the process of identifying the remaining two genes.
In a separate approach, we have analysed the genetic cascade immediately downstream of Twist by testing which of its targets are sufficient to mediate aspects of the morphogenetic activities that depend on Twist. We have shown that in twist mutant embryos the ability of cells to undergo cell shape changes is restored when Snail and Fog are expressed in the mesoderm, but with a considerable delay compared to the wildtype. Thus, Twist acts mainly through its targets snail and fog to induce cell shape changes, but other targets are necessary for the timing of these changes.
FGF signalling mechanisms in the tracheal system:
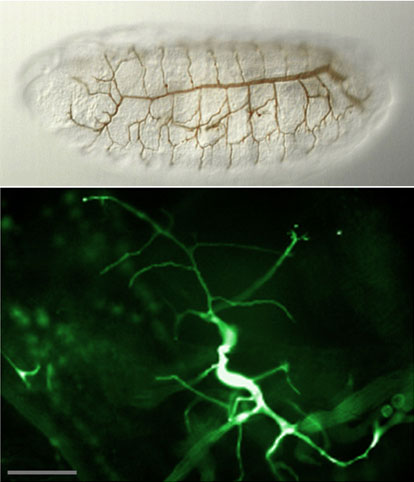
Figure: top: the stereotypic pattern of the embryonic tracheal system. bottom: highly adaptive morphology of a tertiary tracheal cell (GFP) in the L3 larva.
The development of the tracheal system depends on the activity of the Drosophila FGF-receptor Breathless. FGF directs the migrating cells in the embryo and the extension of fine branches of individual tracheal cells in the larva towards cells expressing the FGF-homolog Branchless. FGF receptors are receptor tyrosine kinases (RTK) that activate the MAPK cascade via the small GTPase Ras. However, unlike other RTKs, FGF receptors do not interact directly with the Grb2/sos complex that activates Ras. We have found a cytoplasmic molecule, Dof, that is essential for the transmission of the FGF signal. Dof acts downstream of the FGF receptor (hence the name) and upstream of the Ras and the MAPK cascade. It does not resemble other adaptor or linker proteins in that it lacks structural domains, such as SH2 or PTB domains, typically found in molecules involved in signal transduction. Dof's potential protein interaction domains are a coiled coil region and an ankyrin repeat. Neither of these are essential for function, but we have recently found that a new protein interaction motif, the DBB domain, shared by Dof with two vertebrate signalling proteins from B lymphocytes (BANK and BCAP) is required for Dof's interaction with the FGF receptor and for Dof function in vivo.
Dof interacts with a large number of proteins, including the SUMOylation enzymes Ubc9/semi and PIASy/Su(var)2-10. Dof's DBB domain is also required for this interaction, and it indeed contains a consensus site for SUMOylation, whose modification results in loss of biological function of the protein. Together these findings are a strong indication that SUMOylation of Dof affects FGF signal transmission. In addition, Dof is phosphorylated upon receptor activation, and may be proteolytically cleaved. How these posttranslational modifications affect the activation of Dof, the recruitment of other proteins, and the transmission of the signal from the receptor to its cellular targets is currently being investigated. Genetic screens are being used to identify other components involved in tracheal branch outgrowth.
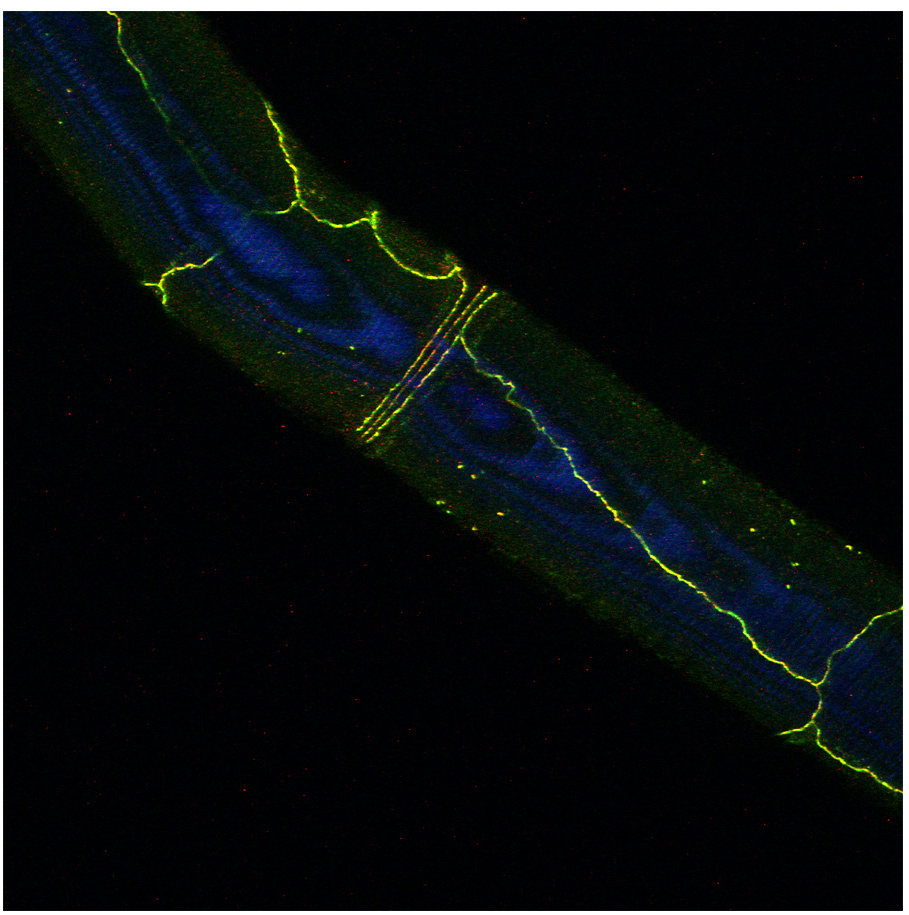
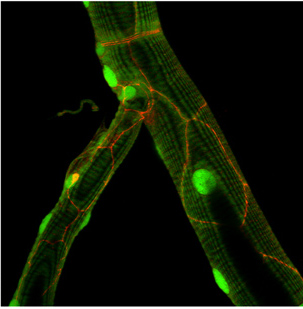
Figure: Drosophila E-Cadherin localization (yellow or red) in dorsal tracheal trunk and in tracheal fusion cells.
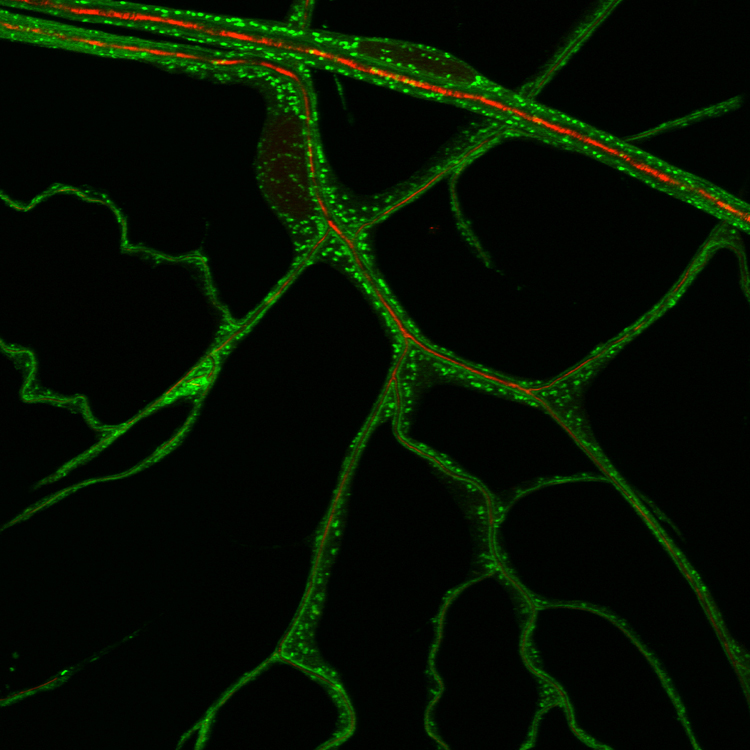
Figure: ER distribution visualized with a KDEL-GFP reporter in terminal tracheal cells.