| SCIENTIFIC BACKGROUND Cyanobacteria are phototrophic prokaryotes, which inhabit environments of widely differering conditions in nearly all habitats on earth. So they are able to survive in freshwater as well as in sea water by adjusting the water potential of the cytoplasm. While the components involved in this acclimatization are characterized (Fig. 1), the regulation of this process is poorly understood. Sensing of salinity changes and fine tuning of de novo synthesis and uptake of compatible solutes are topics we are interested in. |
AIMS Identification of mechanisms involved in stress dependent regulation of gene expression in Synechocystis sp. PCC 6803. Understanding of interaction of transport and de vovo synthesis of the osmoprotective compound glucosylglycerol (GG). Identification and characterization of components involved in regulatory pathways and verification of occurence in other cyanobacteria or eubacteria. |
|
|
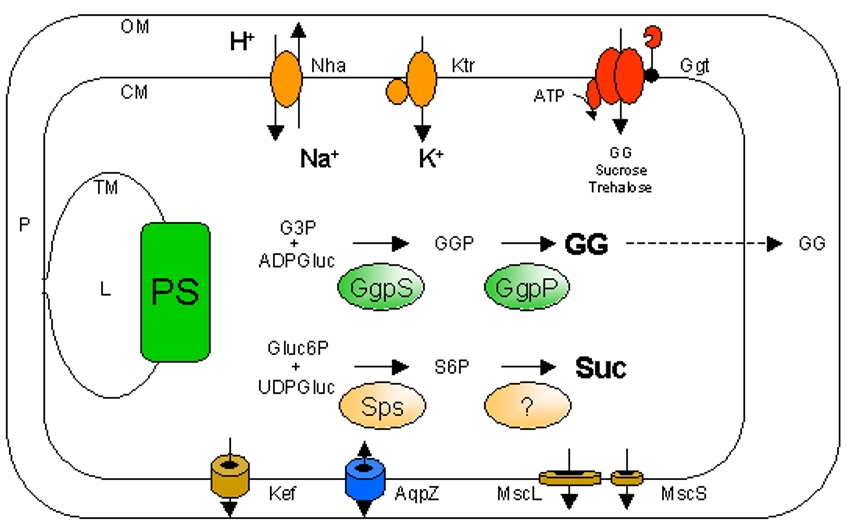
| Shematic drawing of a Synechocystis cell (OM, CM, TM: outer, cytoplasmic and thylakoid membrane; P: periplasm; L: lumen; PS: photosynthesis) Known components involved in ion transport (Nha, Ktr, Kef), osmolyte synthesis (GgpS, GgpP, Sps) or transport (Ggt, AqpZ, MscL, MscS) |
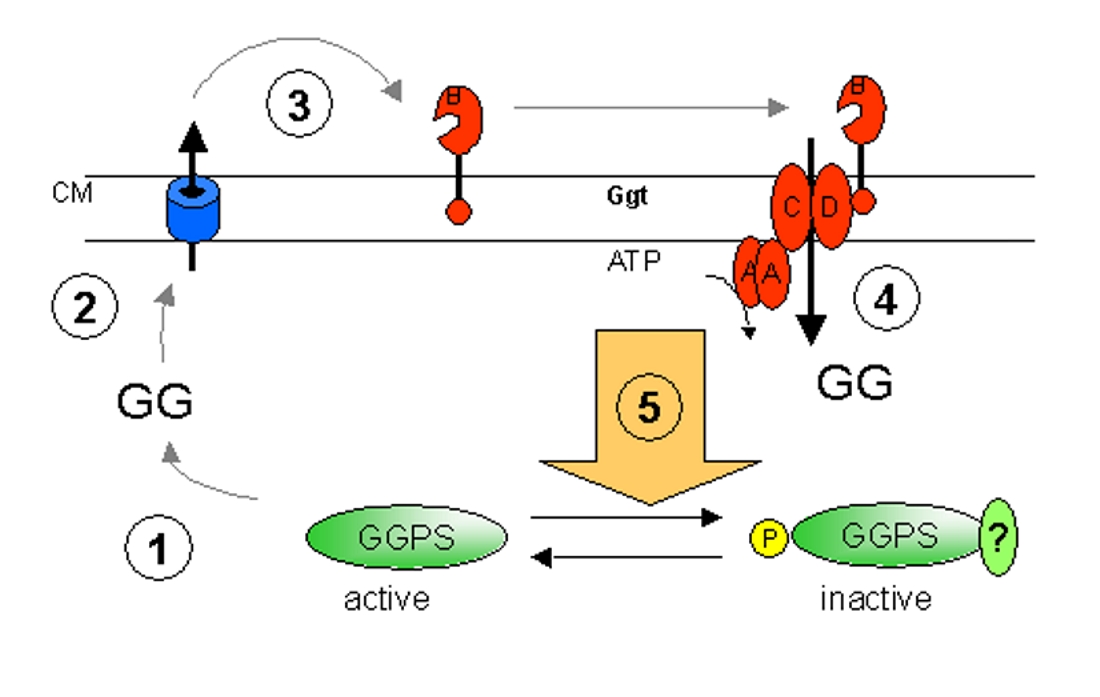
Hypothetical model of fine tuning of GG-synthesis and –uptake. For details see the text. |
|
To characterize the fine tuning of GG-synthesis and GG-uptake we start with a working model (Fig. 2) assuming a regulatory conection of both processes. During the initial phase of salt acclimation enzymes responsible for GG synthesis are activated, so that GG will accumulate in the cytoplasm quickly (1). If the GG content exceeds a concentration necessary to restore the cytoplasmic water potential in dependence on the external salt concentration, cell-turgor will rise resulting in an outflow of GG (2). The occurence of GG in the periplasm will activate the transport machinery (3,4). Finaly this will result in an inhibition of GG-synthesis (5). By overexpression of putative regulatory components of the transport machinery and the key enzyme of GG-synthesis we want to characterize the mechanism in Synechocystis. The interaction will also be characterized biochemically in vitro. Additional regulatory factors can be identified by affinity chromatography with overexpressed proteins or by transposon mutagenesis. In the genome of Synechocystis sp. PCC 6803 42 open reading frames encode for putative histidine kinases. In the lab of Prof. Murata, Okazaki, Japan the collection of all 42 knock out mutants was generated. We analysed the physiological response of all mutants to salt stress. By DNA microarray analysis all salt induced genes of the Synechocystis genome were identified. In a comparative study four Histidine kinases were identified as involved in the perception of salinity changes and responsible for the stress dependent expression of numerous genes. However, additional regulatory mechanisms are present and plant to be identified by bioinformational methods outgoing from the sequence data. (Fig. 3)
|
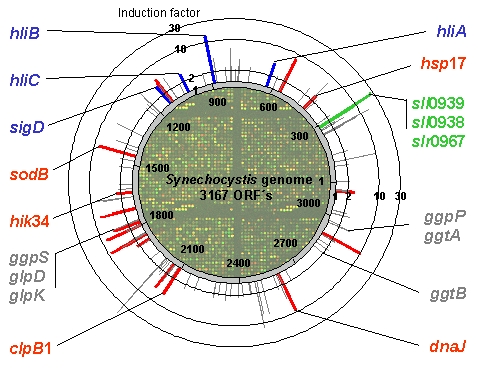
Induction factor and localization of all genes regarded as induced 30 min after salt stress of 684 mM NaCl in the genome of Synechocystis. Gene inductions shown in gray were observed in all Hik-mutants and the WT, whereas inductions shown in red were not observed in the mutant deltaHik34, inductions shown in blue were not observed in deltaHik33 and inductions shown in green were not observed in deltaHik16 and deltaHik41. |
| FUNDING
|
COOPERATION |
| SELECTED
PAPERS [Abstract] [Abstract] [Abstract] [Abstract] [Abstract] |
| Weitere Informationen
Verbreitung von Cyanobacterien Der Modellstamm Synechocystis sp. PCC 6803. |