Projects >Stress Signaling in Disease
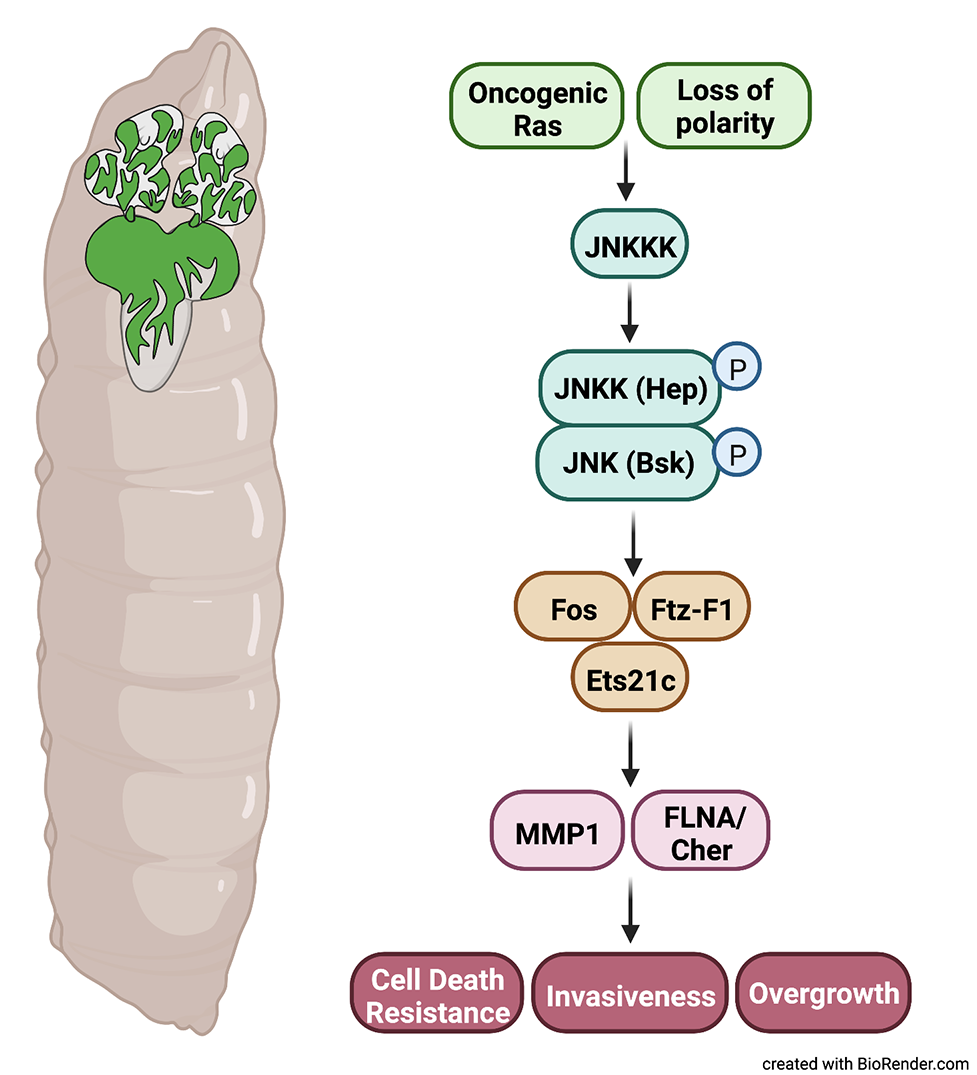
Our interest has been focused on elucidating the complex role of the stress-inducible Jun-N-terminal kinase (JNK) pathway during malignant transformation using the Drosophila tumor model based on the cooperation of oncogenic Ras and loss of polarity. By combining loss- and gain-of-function oncogenic events in visibly marked clonal tumors introduced into otherwise wild-type imaginal epithelium, we showed that the contribution of JNK signaling to tumor formation varied depending on the genotype of the tumor and also on the strength of the JNK signal (Uhlirova et al., 2005). We further demonstrated the requirement of a matrix metalloproteinase (MMP) downstream of JNK for the spreading of highly malignant tumors to secondary sites (Uhlirova and Bohmann, 2006). We provided genetic evidence that the malignancy of polarity-deficient, Ras-driven tumors in Drosophila epithelia requires the cooperation of three transcription factors of distinct families, namely Fos, Ets21c, and Ftz-F1, all acting downstream of JNK signaling (Kulshammer et al., 2016).
Our recent work provided novel insights into how tumor cells in vivo exploit
the mechanosensitive cellular machinery to promote malignancy. We highlighted a crucial role of an actin croslinker FilaminA/Cheerio (Cher) as a JNK-regulated mechanosensitive scaffold that acts in concert with its interacting partners within the cell cortex to regulate Hippo/Yki signaling, promoting tumor cell contractility, growth, and invasiveness (Kulshammer et al., 2013, Kulshammer et al., 2022).