Prof. Dr. Gerd Meyer, Dipl.-Chem., Dr. rer. nat.
Inorganic Solid State and Coordination Chemistry:
Synthesis, Crystal and Electronic Structures, Properties
Universität zu Köln, Department für Chemie, Institut für Anorganische Chemie, Lehrstuhl I, Greinstraße 6, D-50939 Köln, Germany
Tel.: (+49)221 470 3262
Fax: (+49)221 470 5083
E-Mail: gerd.meyer@uni-koeln.de
http://www.gerdmeyer.de
Curriculum Vitae
-
Born 1949 at Schadeck (Hessen, Germany)
-
Diplom in Chemistry 1972 (Justus-Liebig-Universität Gießen)
-
Dr. rer. nat. 1976 (Justus-Liebig-Universität Gießen):
„Beiträge zur Chemie ternärer Oxide der Alkalimetalle mit leichten und schweren Übergangsmetallen“ mit einem Anhang über „Effektive Koordinationszahlen, ECoN, ein einfaches Hilfsmittel bei der Beschreibung und Charakterisierung von Kristall-strukturen“ -
Postdoc with Professor John D. Corbett, Iowa State University, Ames, Iowa, USA, 1980
-
Habilitation 1982 (Justus-Liebig-Universität Gießen):
„Beiträge zur Synthese und Kristallchemie der komplexen Halogenide“ -
Akademischer Rat 1977-1988 (Justus-Liebig-Universität Gießen)
-
Privat-Dozent 1982-1988 (Justus-Liebig-Universität Gießen)
-
Professor (Chair, C4) 1988-1996 (Universität Hannover)
-
Professor (Chair, C4) 1996- (Universität zu Köln)
Awards & Honours
-
Preis der Justus-Liebig-Universität Gießen 1987
-
Carl-Duisberg-Gedächtnispreis (Gesellschaft Deutscher Chemiker, GDCh) 1987
-
Gold Medal of the University of Wroclaw (Poland) 2000
-
Terrae Rarae Award 2005
-
Member, Stiftungsrat, Bayer Science Foundation 2007
-
Fellow, Royal Society of Chemistry 2009
-
Frank H. Spedding Award 2011
-
Editor-in-Chief, Crystals, 2011-
-
Jubiläumsmedaille der Uniwersytet Wroclaw 2012
-
Adjunct Professor, The University of Alabama, Tuscaloosa, AL, USA, 2012-
Bibliography: Books
-
“Synthesis of Lanthanide and Actinide Compounds”, G. Meyer, L. R. Morss, Kluwer Academic Publishers, Dordrecht, NL (1991).
-
"Allgemeine und Anorganische Chemie" (two volumes), H.R. Christen, G. Meyer, Salle + Sauerländer (1994).
-
"Grundlagen der Allgemeinen und Anorganischen Chemie", H.R. Christen, G. Meyer, Salle + Sauerländer (1997).
-
"Inorganic Chemistry Highlights", G. Meyer, D. Naumann, L. Wesemann (co-editors), Wiley-VCH (2002).
-
"Inorganic Chemistry in Focus", G. Meyer, D. Naumann, L. Wesemann (co-editors), Wiley-VCH. Vol. II, 2005; Vol. III, 2006.
-
Co-Editor of the Textbook Series "Inorganic Chemistry", Wiley Chichester (12 volumes, 1993 to present)
Bibliography: Review Articles & Highlights
-
The Synthesis and Structures of Complex Rare-Earth Halides — Prog. Solid State Chem. 1982, 14, 141-219.
-
Reduced Halides of the Rare-Earth Elements — Chem. Rev. 1988, 88, 93-107.
-
Thermal behaviour of complex halides — Eur. J. Solid State Inorg. Chem. 1991, 28, 1209-1243.
-
Unusual Valences in Rare-Earth Halides — Chem. Mater. 1992, 4, 1157-1168.
-
The Ammonium Ion for Inorganic Synthesis — in: Advances in the Synthesis and Reactivity of Solids (T. E. Mallouk, ed.), JAI Press, Greenwich, Connecticut, USA, 1994, 2, 1-26.
-
The Alkali-Poor Part of the Pseudoternary Triangle AX/BX2/MX3: Crystal Structures, Properties, and Potentials of (Alkali)/Alkaline-Earth/Rare-Earth Chloride Materials — Chem. Mater. 1998, 10, 2994-3004.
-
Simple and Complex (Rare Earth) Halides — Handbook on the Physics and Chemistry of Rare Earths 2000, 28, 53-129.
-
Affinity of Divalent Mercury Towards Nitrogen Donor Ligands — Z. Anorg. Allg. Chem. 2003, 629, 147-1461.
-
Divalent Scandium — Inorg. Chem. in Focus (G. Meyer, D. Naumann, L. Wesemann, eds.), Wiley-VCH, Weinheim, 2005, 2, 105-120.
-
Forty-five Years of Praseodymium Di-iodide, PrI2 — Inorg. Chem. in Focus (G. Meyer, D. Naumann, L. Wesemann, eds.), Wiley-VCH, Weinheim, 2006, 3, 45-60.
-
The Reduction of Rare-Earth Metal Halides with Unlike Metals – Wöhler’s “Metallothermic Reduction” — Z. Anorg. Allg. Chem. 2007, 633, 2537-2552.
-
The Oxidation of Metals with Liebig Acids — Z. Anorg. Allg. Chem. 2008, 634, 201-222.
-
Superbulky Ligands and Trapped Electrons: New Perspectives in Divalent Lanthanide Chemistry — Angew. Chem. Int. Ed. 2008, 47,4962-4964.
-
Cluster Complexes as anti-Werner Complexes — Z. Anorg. Allg. Chem. 2008, 634, 2729-2736.
-
Coordinative Flexibility of Monovalent Silver in [AgI←L1]L2 Complexes — in Design and Construction of Coordination Polymers (Mao-Chun Hong, Ling Chen, editors), John Wiley and Sons, 2009, chapter 1, 1-23.
-
Halides of Titanium in Lower Oxidation States — Z. Anorg. Allg. Chem. 2009, 635, 1497-1509.
-
Heteroleptic Samarium(II) Complexes by Base-Induced Reduction — Angew. Chem. Int. Ed. 2010, 49, 3116-3118.
Research: Rare-Earth and Transition Metal Solid State
and Coordination Chemistry
Transition (d block) and rare-earth (f block) metal halide
chemistry is rich of oxidation states. Of special interest are such
that are lower than the respective group number. Elaborate synthetic
routes have been developed to explore the respective systems and to
grow crystals for structure determination. The next step is the elucidation
of routes to pure substances for physical property evaluation.
Except for the classic conproportionation route, we have further developed
The Reduction of Rare-Earth Metal Halides with Unlike Metals –
Wöhler’s “Metallothermic Reduction”. Metal halides
(such as PrI3) are reduced with alkali metals yielding not
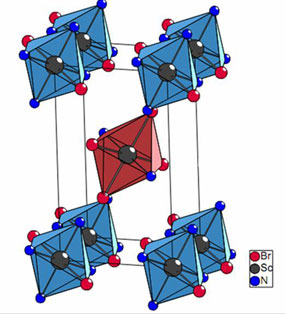
only binary halides (as PrI2 and Pr2I5) but also cluster complexes (Cs4Pr6I13C2,
with some carbon in the reaction mixture).
These Cluster Complexes are in fact anti-Werner Complexes when re-written
as, for example, {(C2)Pr6}I13Cs4.
A dicarbon unit is encapsulated in a praseodymium octahedron (“cluster”)
which is surrounded by and connected through iodine ligands with additional
cesium atoms filling space and contributing electrons.
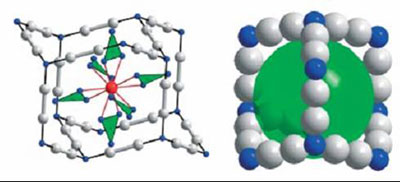
Cluster complexes may be molecules as in {Sc4C10Sc20}I30,
consisting of a truncated hollow T4 supertetrahedron of iodine stuffed
with a T3 supertetrahedron of scandium that encapsulates the adamantoid
cluster Sc4C10.

In most cases, however, they qualify as extended solids
with various dimensionalities. Oligomeric cluster complexes are observed
in {Ru5La24}Br39, with a pentagon of
ruthenium atoms included in a bicapped hexagonal prismatic cluster of
lanthanum atoms.
The alternative to oligomers are chains, as seen, e.g., in {Os5Lu20}I24
with eight-coordinate endohedral osmium atoms.
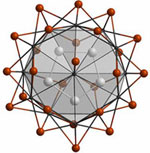

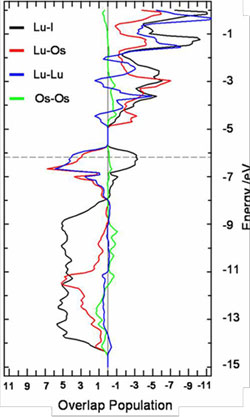
The electronic structures of these anti-Werner cluster complexes, {ZRx}Xz, are such that the main bonding interactions are of the endohedral atom—cluster atoms, Z—R, as well as cluster-atoms—(halide)ligands, R—X, type with little Z—Z (if at all possible) and R—R bonding. This can be clearly seen from band structure calculations and the overlap populations derived therefrom.
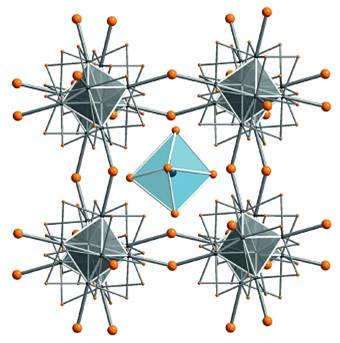
Transition metal halides in low oxidation states are by no means understood.
This becomes obvious by the crystal structure of “TaBr3”
which was only recently determined. It is in fact TaBr2.94
= [{Ta6}Br15][TaBr6]0.86
exhibiting a perovskite-related arrangement with the [{Ta6}Br12Br6/2]
framework encapsulating a [TaBr6] octahedra.
Except for Wöhler’s Metallothermic Reduction
a second synthetic route has been extraordinarily prolific throughout
the years, The Oxidation of Metals with Liebig Acids.
Non-noble metals may be oxidized by any compound with an acid hydrogen
atom (a Liebig acid, by definition). There is a large variety of organic
compounds with HO– or HN– functions with acid constants
that allow for substitution of the hydrogen atom by a metal atom. We
have especially explored carboxylic acids yielding a large variety of
new simple and complex salts, mostly coordinationation polymers.
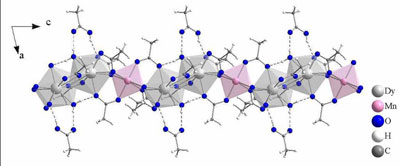
These include alkanoates as the propionate Mn(OPr)2 (above)
as well as benzoates like Mn19(OBz)38(HOBz)2,
anhydrous acetates like Nd(OAc)3 or the complicated [Eu2(OAc)6(HOAc)2(H2O)2]-(HOAc)4
or, most importantly, heterobimetallic complexes such as [Dy2Co(OAc)4(H2O)2](HOAc)2.
The ammonium ion, (NH4)+, represents a special Liebig acid. It is available in a large variety of salts and may react as an acid/oxidant or as a (base)/reductant and is also a pseudo alkali-metal cation. Again, a large variety of compounds has been obtained by reaction of (NH4)X (X = F, Cl, Br) with non-noble metals, of which (NH4)6[Ta5(NH)4Cl17] and [Fe(NH3)6]3[Fe8Br14], are perhaps the most spectacular ones. Rare-earth metal compounds such as ScBr3.3NH3 = [Sc(NH3)4Br2][Sc(NH3)2Br4] or (NH4)GdF4 (with nine-coordinate Gd(III)) are easily available by this general route.

These reactions led us also to the coordination chemistry of transition metal ions such as Hg(II) and Ag(I), both closed shell d10, Ni(II) (d8), Mn(II) (d5) with (organic) N-donor ligands, as well as O- and S-donors. The Affinity of Divalent Mercury Towards Nitrogen Donor Ligands was widely studied. Hg(I) as [Hg—Hg]2+ dumbbells are also important. The new cristobalite like cage structures as in [(Hg2)2O][Pb(NO3)3]2 are especially noteworthy.
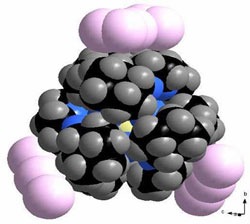
Recently we have focused on the application of crown ethers to, first, encapsulate transition metal ions such as Ag+ and, secondly, to provide large cations as counter-ions for networks of polyiodides and polyinterhalides. As an example for the first one serves [Ag2(db24c8)(Tfa)2], a molecular complex with two Ag+ ions encapsulated in dibenzo-24-crown-8, bridged by triflate anions above and below, such that the Ag+—Ag+ distance is only 290.45(7) pm. A spectacular example of a poly-interhalide is the planar [Cl(I2)4]-; layers of these anions and layers of the hitherto unknown cations [(H5O2)(I2b15c5)2]+ are stacked in the crystal structure.
Rare-earth cations encapsu-lated in crown ethers excellently serve as templates for polyiodide networks. Traces of water or similar oxygen-donors occupy the coordination sphere of the rare-earth cations more efficiently than crown ethers which is documented by the compounds [LaI(db18c6)(dme)](I5)2 or the spectacular [Lu(H2O)3(db18c6)(thf)6]4(I3)2(I5)6(I8)(I12). Larger amounts of water lead to a variety of double-shell complexes, for example [R(H2O)9(dch18c6)3](I3)3 with R the whole series of the rare-earth elements.

![]()
Prof. Dr. Gerd Meyer - www.gerdmeyer.de
| Universität zu Köln
| zum Seitenanfang
