|
|
Experimental and theoretical investigations on aromatic peroxides
|
|
|
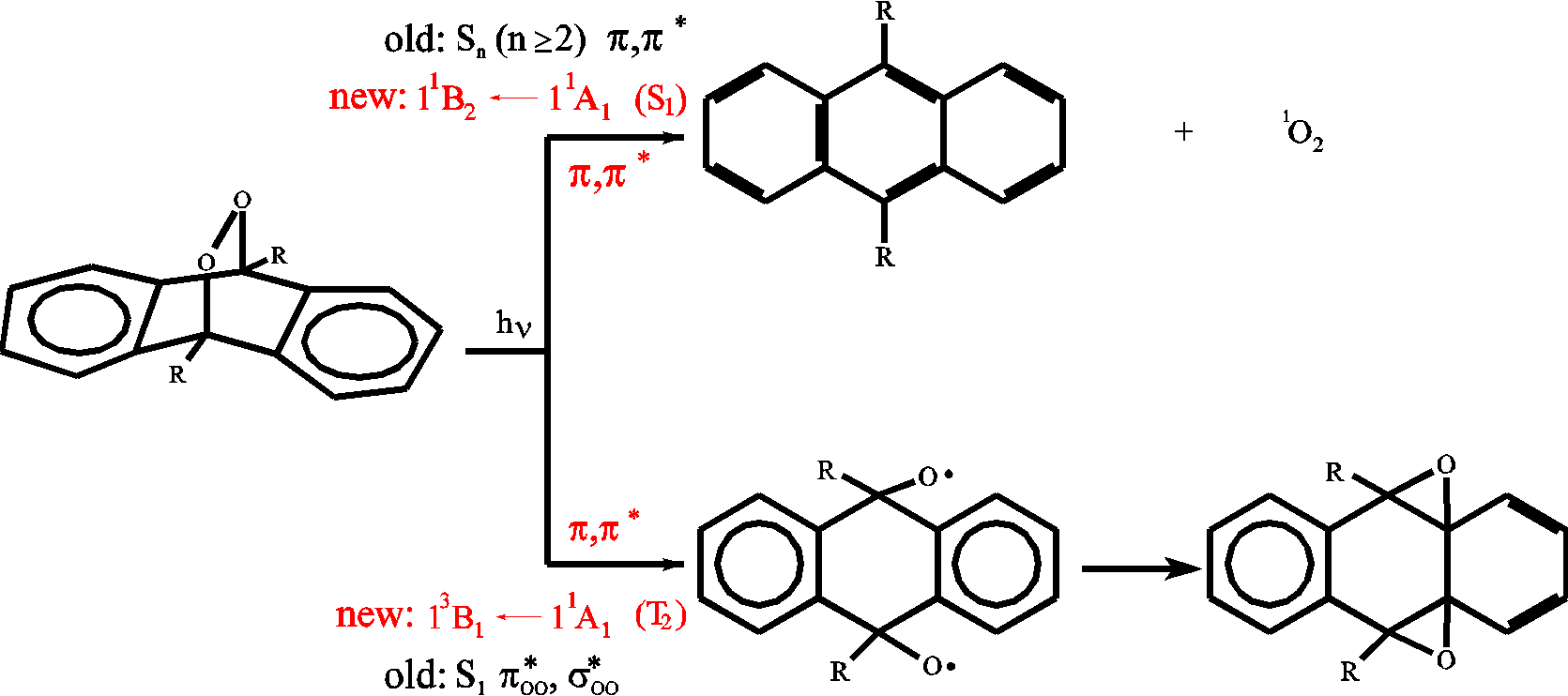
Endoperoxides of ortho-fused benzenes like naphtalene and
anthracene are of fundamental interest due to their
photochemical behavior. When such molecules are excited
lambda=270 nm cycloreversion to singlet oxygen and the
aromat in its ground state occurs. Excitation at lambda
> 435 nm induces the homolytic cleavage of the peroxide
bridge (see figure). Until recently it was assumed that
cycloreversion occurs from a higher excited state
(Sn >= 2) whereas excitation into the
S1 state leads to cleavage of the peroxide bridge.
This assignment contradicts Kasha's rule wich states that
efficient photochemical reactions should occur from the
first excited singlet state. Experimental and
theoretical investigations show that at least
9,10-anthracenendoperoxid and
9,10-dimethylanthracenendoperoxide are no exceptions of
Kasha's rule. The S1 state is located at ca. 34000
cm-1 (old assignment: S2) and the
S2
state is located at ca. 23000 cm-1 (old
assignment: S1).
|
|
|
|
|
|
Experimental Investigations on 1:1 Contact Complexes
(Dr. M. Kalb)
|
|
|
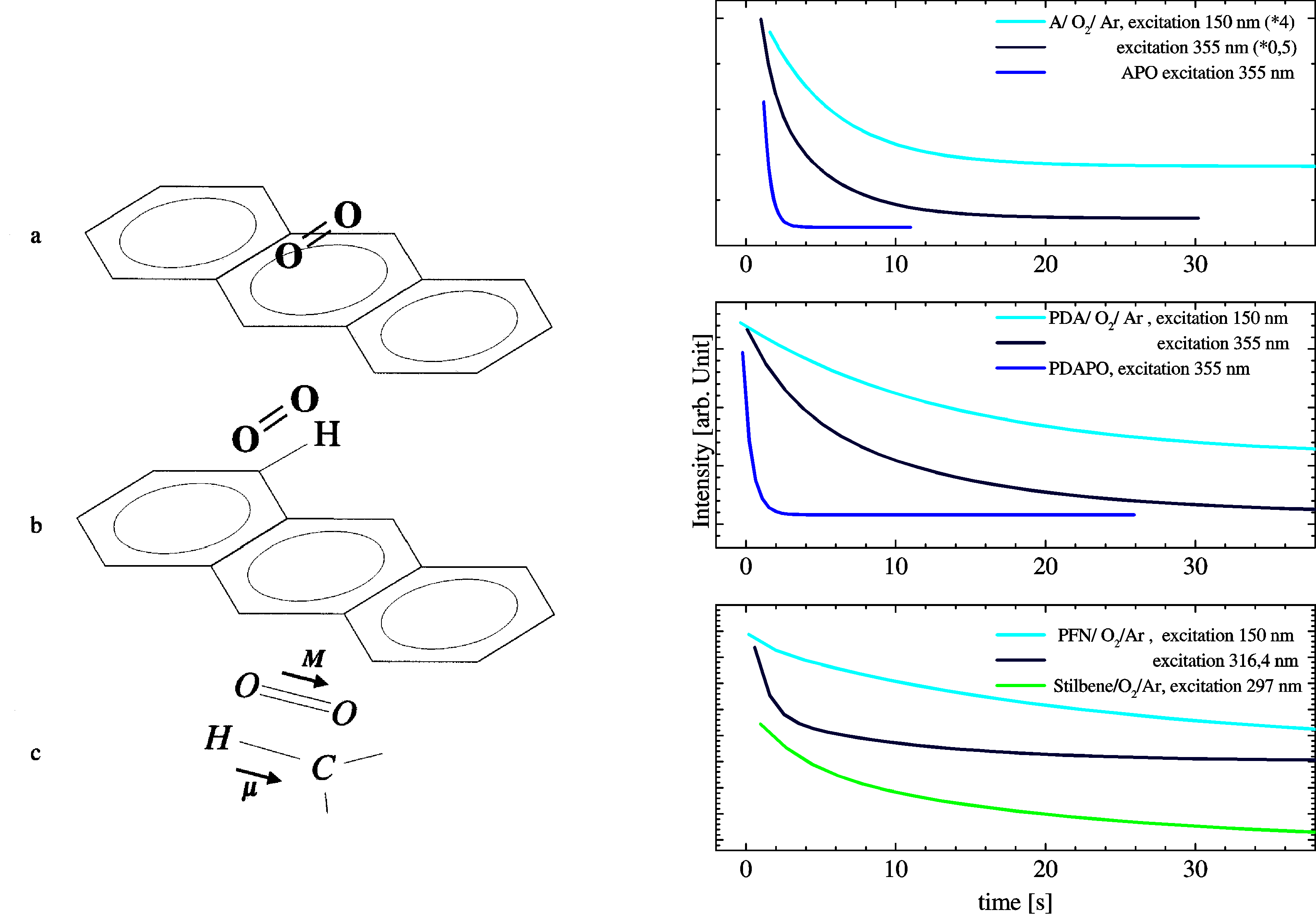
Laser photolysis (275 nm) of anthracene endoperoxides in an
argon matrix leads through cycloreversion to 1:1 contact
complexes which consist of singlet oxygen and the
aromat. The matrix cage prohibits the diffusion of the
photofragments so that molecular interactions and
energy transfer processes can be studied in a well
definded spatial environment.
The lifetime of singlet oxygen in 1:1 contact
complexes after excitation of the aromat followed by
energy transfer is significiantly shorter as in a
corresponding experiment in statistical distributet matrices.
This effect can be explained through efficient
quenching processes between the aroamt and the oxygen
molecule which are based on electron-to-electron (e-e)
and electron-to-vibrational (e-v) energy
transfer. This energy transfer depends on the spatial
configuration and the concentration of the oxygen molecule.
|
|
|
|
|
|
Experimental investigation of the photochemical induced
energy transfer after photolysis of three-atomic
molecules
|
|
|
The mechanismn of the photochemical induced energy
transfer could be observed for the first time at the
CO2 molecule. The aim of this project is to
probe if this mechanism can be applied to other three
atomic linear and non linear molecules. These
experiments are currently carried out at the BESSY
synchrotron in Berlin.
|


 Andreas Klein
Andreas Klein 