Projects > Stress Signaling and Homeostasis
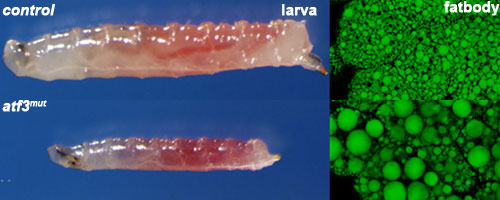
The integration of metabolic and immune responses is an essential aspect of homeostasis. The ability to withstand starvation and mount an effective defense against pathogens is a fundamental prerequisite for organismal survival. Our lab uses the Drosophila model to better understand the underlying molecular mechanisms linking metabolic and immune system homeostasis. We have identified a role for the Drosophila basic-leucine zipper (bZIP) protein, Activating transcription factor 3 (Atf3), in maintenance of metabolic and immune system homeostasis during fly development. (Rynes et al., 2012, Mol Cell Biol). Loss of Atf3 results in chronic inflammation and starvation responses mounted primarily by the larval gut epithelium, while the fat body suffers lipid overload, causing energy imbalance and death. Pro-inflammatory and stress-response signaling through NF-kB/Relish, Jun-N-terminal kinase (JNK) and the transcription factor FOXO are hyperactive in atf3 mutants, causing deregulation of genes important for immune defense, digestion and lipid metabolism. Reducing the gene dose of either FOXO or Relish normalizes both the lipid metabolism and gene expression profiles in atf3 mutants. Function of Atf3 is conserved between flies and mammals, as human ATF3 averts some of the Drosophila mutant phenotypes, improving their survival.