Public Outreach
Mitochondria are little compartments within a cell that produce the energy needed for most biological processes. Each cell possesses several mitochondria, which can fuse together and then break again into smaller units. This fusion process is essential for cellular health.
The key gatekeeper of the fusion mechanism is a protein called mitofusin, which locates to mitochondria. In humans, improper regulation of mitofusins causes an incurable disease of the nerves and the brain called Charcot-Marie-Tooth 2A. Understanding how the fusion of mitochondria is controlled can lead to new drug discoveries.
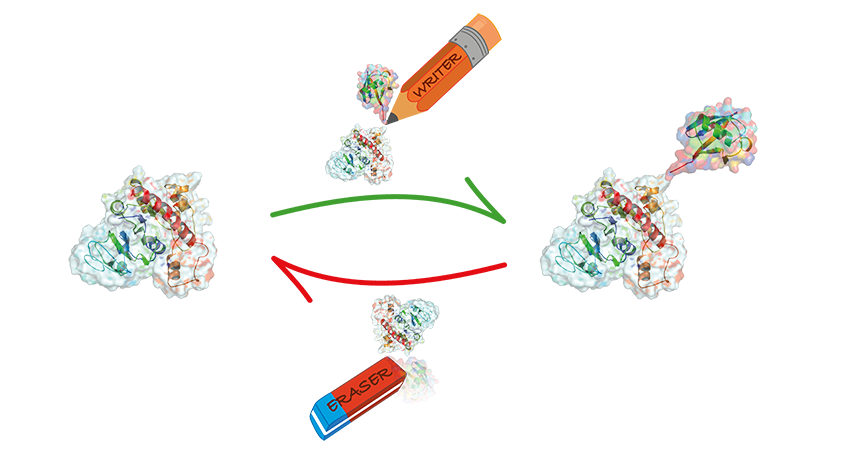
Cells often ‘tag’ proteins with small molecules, called ubiquitin, to change the protein’s role and how it interacts with other cellular structures. This process, called ubiquitylation, is carried out by enzymes called ligases (ubiquitin “writers”) and reversed by editing enzymes called deubiquitylases (ubiquitin “erasers”).
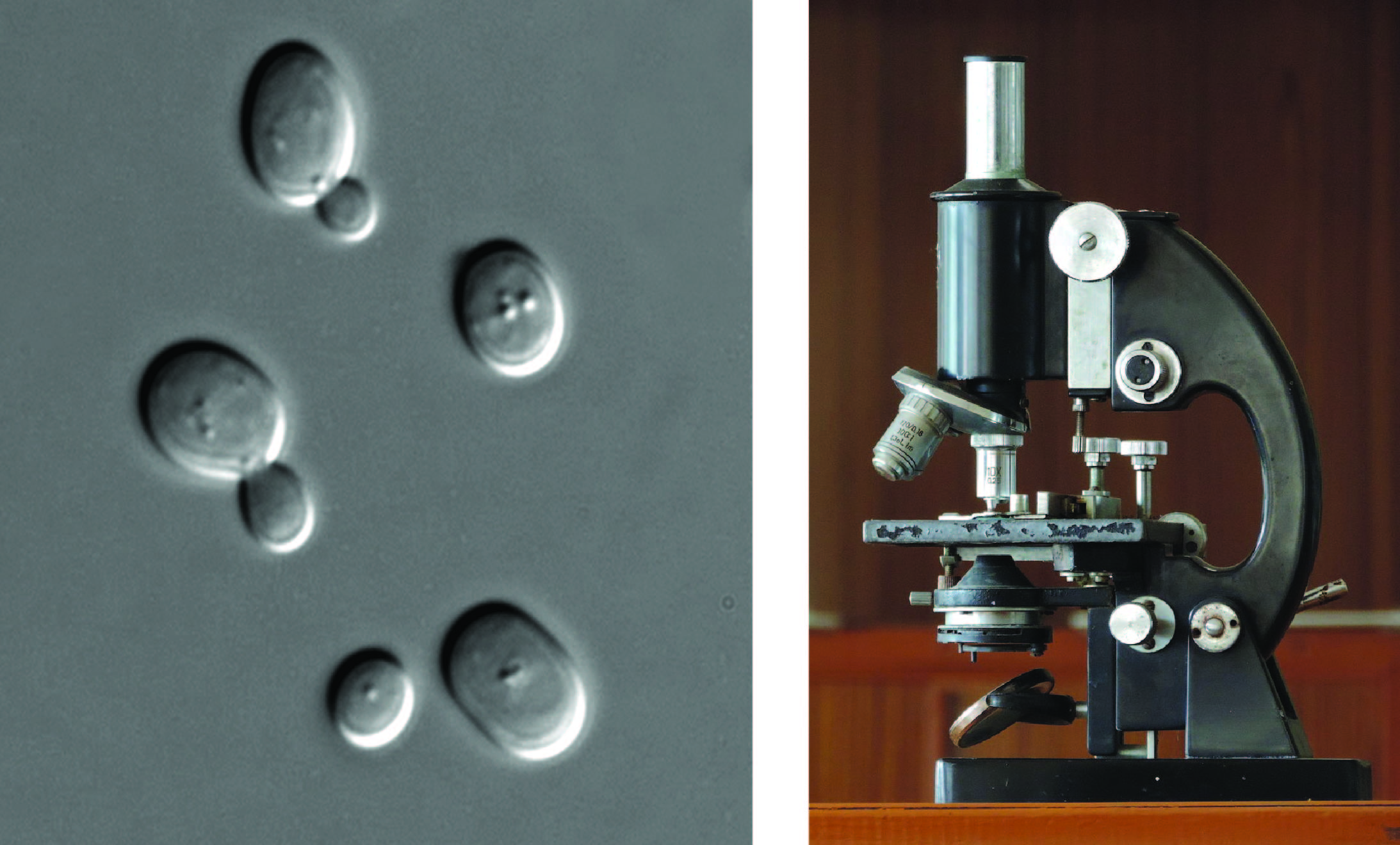
Depending on how they are ‘tagged’, mitofusins can exist in two forms. One type of tagging means that the protein then promotes fusion of the mitochondria; the other leads to the mitofusin being destroyed by the cell. Mitofusins in the baker’s yeast are very similar to the ones in people, so that a simpler organism like yeast simplifies studying how ubiquitin regulates mitochondrial fusion. Yeast are organisms with only one very small cell that can only be seen using a microscope.
Our group has identified two “eraser” proteins in the cell with a major role in controlling mitochondrial fusion: Ubp12 and Ubp2. Ubp12 prevents fusion, while Ubp2 activates it. These molecules carry out their roles by acting on mitofusin. Ubp2promotes fusion by attaching to the mitofusin that is labeled to be destroyed, and removing this tag: the mitofusin will then not be degraded, and can promote fusion. Ubp12 prevents fusion by removing the ‘pro-fusion’ tag on the mitofusin that prompts mitochondrial fusion.
But how does the cell regulate Ubp12 and Ubp2, meaning how does it decide to activate or inhibit mitochondrial fusion? We know now Ubp12 regulates Ubp2, meaning that activation and inhibition of fusion are coordinated. This is possible because Ubp12 and Ubp2 are themselves ‘tagged’ with ubiquitin, meaning that the predator also turns into the prey. In turn, the experiments reveal that a master protein called Cdc48 can control the entire Ubp12- Ubp2-mitofusin pathway. Cdc48 directly represses Ubp12 and therefore its anti-fusion activity. This inhibition also leaves Ubp2 free to stimulate fusion through its action on mitofusin.
In summary, Ubp2, Ubp12 and Cdc48 fine-tune the ubiquitylation status of yeast mitofusin and thereby modulate mitochondrial fusion, critical for healthy mitochondrial function and cellular integrity.




