Ubiquitylation
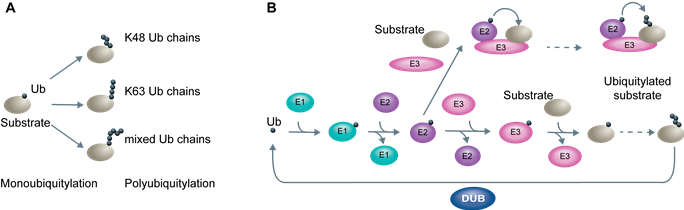
From yeast to mammals, mitochondrial fusion is regulated by post-translational modifications – like ubiquitylation - of its central component, mitofusin. Ubiquitin, a 76-small amino acid protein, can be covalently attached to lysine residues of target proteins as a single moiety, or in the form of different ubiquitin chains. This leads to distinct cellular functions, from protein degradation to stabilization of protein complexes. Ubiquitylation begins with the activation of the modifier by E1 enzymes, followed by its transfer to E2 conjugating enzymes, and its ligation to the target substrate by E3 ubiquitin ligases, like the SCF complexes, where the variable F-box subunit mediates substrate recognition.
Protein post-translational modification by ubiquitylation. (A) Different possibilities of post-translational modification of target substrates by monoubiquitylation or subsequent polyubiquitylation. (B) Enzymatic cascade allowing ubiquitylation. Ubiquitin is successively transferred from enzymes E1 to E2. Then, ubiquitin is either directly transferred to the E3 before its final conjugation with the substrate or, alternatively, transferred from the E2 to the substrate brought into its proximity by the E3.